Microsoft word - regenerative endo, mso, 2009, handout.doc
IT'S ALIVE: REVASCULARIZATION OF NECROTIC PULP TISSUE IN IMMATURE TEETH Background Reference: Regenerative Endodontics: A Review of Current Status and a Call for Action, Peter E. Murray, Franklin Garcia-Godoy, and Kenneth M. Hargreaves, J Endod, 2007 Background • Approximately $400 billion spent treating Americans suffering some type of tissue loss or end-stage organ o 20,000 organ transpl
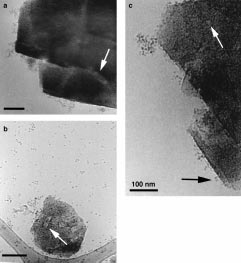 of solutions containing proteins interacting with zeolite Y
crystals. Pronounced adsorption of ferritin on ultrastable
zeolite Y crystals is shown to be correlated to protein
aggregation. Adsorption of ferritin molecules on low- and
high-silica zeolite Y crystals results in different arrangements
of the protein molecules. It is also shown that structural
information, like unit cell parameters, can be obtained from
inorganic materials present in vitrified solutions.
of solutions containing proteins interacting with zeolite Y
crystals. Pronounced adsorption of ferritin on ultrastable
zeolite Y crystals is shown to be correlated to protein
aggregation. Adsorption of ferritin molecules on low- and
high-silica zeolite Y crystals results in different arrangements
of the protein molecules. It is also shown that structural
information, like unit cell parameters, can be obtained from
inorganic materials present in vitrified solutions.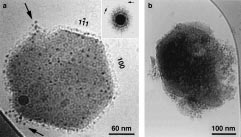 large protein aggregates, some containing 50 ± 100 molecules.
large protein aggregates, some containing 50 ± 100 molecules.