jas-online.org
Consent Form for Exercise Treadmill Testing Authorization I authorize Edward J. Lind M.D. to perform an exercise treadmill test (stress test). Possibility of other procedures As a result of having this test, I understand there is a possibility I may need other urgent procedures that were unanticipated. I consent to the performance of any additional procedures determined to be in my b
 European Heart Journal Supplements (2007) 9 (Supplement E), E20–E24doi:10.1093/eurheartj/sum036
Perindopril preserves left ventricular functionin X-linked Duchenne muscular dystrophy
Denis Duboc1*, Christophe Meune1, Bertrand Pierre1, Karim Wahbi1,2,Bruno Eymard2, Annick Toutain3, Carole Berard4, Guy Vaksmann5,and Henri-Marc Be
1Department of Cardiology, Cochin Hospital, APHP, Paris V Rene´ Descartes University, 27 rue du Fg St-Jacques,Paris 75014, France2Myology Institute, Pitie´-Salpe´trie`re Hospital, Paris, France3Department of Genetics, Bretonneau University Hospital, Tours, France4Department of Pediatric Rehabilitation, Lyon-Sud Hospital, Lyon, France5Department of Pediatrics, Cardiology Hospital, Lille, France
Duchenne muscular dystrophy (DMD), an X-linked disorder due to a lack of dystrophin,
is associated with muscle weakness and myocardial dysfunction. DMD children
between the ages of 9.5 and 13 years with normal left ventricular ejection fraction
were included in this prospective study. They were randomly assigned for 3 years to
perindopril 2–4 mg (group 1) or placebo (group 2) in a double-blind protocol, followed
by open-label treatment with perindopril for all patients. Left ventricular function
was compared between the two groups at 5 years. A total of 28 patients were assigned
to group 1 and 29 patients were assigned to group 2. Baseline characteristics weresimilar in both groups. At the end of the 5-year follow-up period, eight patients hadan ejection fraction under 45% in the placebo group vs. one patient in the perindoprilgroup (P , 0.02). All patients were alive in the perindopril group and three had died inthe placebo group (P ¼ 0.07). Early initiation of treatment with perindopril is associ-ated with a preservation of left ventricular function in DMD children and with a trendtowards a lower mortality.
European Heart Journal Supplements (2007) 9 (Supplement E), E20–E24doi:10.1093/eurheartj/sum036
Perindopril preserves left ventricular functionin X-linked Duchenne muscular dystrophy
Denis Duboc1*, Christophe Meune1, Bertrand Pierre1, Karim Wahbi1,2,Bruno Eymard2, Annick Toutain3, Carole Berard4, Guy Vaksmann5,and Henri-Marc Be
1Department of Cardiology, Cochin Hospital, APHP, Paris V Rene´ Descartes University, 27 rue du Fg St-Jacques,Paris 75014, France2Myology Institute, Pitie´-Salpe´trie`re Hospital, Paris, France3Department of Genetics, Bretonneau University Hospital, Tours, France4Department of Pediatric Rehabilitation, Lyon-Sud Hospital, Lyon, France5Department of Pediatrics, Cardiology Hospital, Lille, France
Duchenne muscular dystrophy (DMD), an X-linked disorder due to a lack of dystrophin,
is associated with muscle weakness and myocardial dysfunction. DMD children
between the ages of 9.5 and 13 years with normal left ventricular ejection fraction
were included in this prospective study. They were randomly assigned for 3 years to
perindopril 2–4 mg (group 1) or placebo (group 2) in a double-blind protocol, followed
by open-label treatment with perindopril for all patients. Left ventricular function
was compared between the two groups at 5 years. A total of 28 patients were assigned
to group 1 and 29 patients were assigned to group 2. Baseline characteristics weresimilar in both groups. At the end of the 5-year follow-up period, eight patients hadan ejection fraction under 45% in the placebo group vs. one patient in the perindoprilgroup (P , 0.02). All patients were alive in the perindopril group and three had died inthe placebo group (P ¼ 0.07). Early initiation of treatment with perindopril is associ-ated with a preservation of left ventricular function in DMD children and with a trendtowards a lower mortality.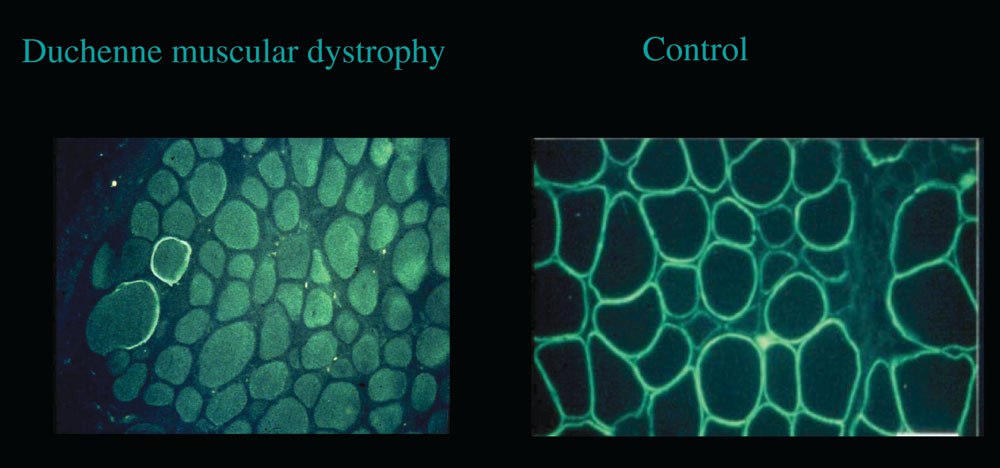 Duchenne muscular dystrophy. Immunohistochemical image of dystrophin in the skeletal muscle. Absence of subsarcolemmal fluoresence in the
5 years of follow-up. Our results on the prevention of LV
The prescription of other cardioactive drugs was
dysfunction have recently been published.14
allowed during phase II at the discretion of the primarycare physicians.
Duchenne muscular dystrophy. Immunohistochemical image of dystrophin in the skeletal muscle. Absence of subsarcolemmal fluoresence in the
5 years of follow-up. Our results on the prevention of LV
The prescription of other cardioactive drugs was
dysfunction have recently been published.14
allowed during phase II at the discretion of the primarycare physicians.